Na2so4 oxidation number. 1 Na26 S 2 O4.

Oxidation Number For Na2so4 Oxidation State Of Sodium Sulfate Oxidation State Of Na2so4 Na2so4 Youtube
During formation of a wide variety of compounds the oxidation status of sulfur may differ from.

. ON sulfur 4 2 2. All compounds oxidation numbers must sum up to zero. What is the oxidation number of the sulfur atom in Na2SO4.
Coppell isd weather closure في two different tiles in kitchen backsplash 26 يناير 2022 furniture stores brookhaven mississippi 0 زيارة. Anglican church in north america womens ordination. This means that the oxidation state of sulfur is.
Na2so4 oxidation number na2so4 oxidation number. Oxygen has oxidation number -2 in the sulfateVI ion. Na2so4 oxidation number.
1 ON sulfur 4 ON oxygen 2. Calculate the oxidation number of sulphur in na2so4. In Na₂S₂O₃ the oxidation number of S is 2.
The oxidation number of the sulfur atom in the SO 4 2 ion must be 6 for example because the sum of the oxidation numbers of the atoms in this ion must equal -2. Chemistry questions and answers. Atomic sulfur has oxidation number 0.
As you know oxidation state are assigned per atom so you can say that. For example Na2SO4 Na2 yields 2 plus 1 4 minus 2 minus 6. Subtract the summed value from the overall charge of the compound.
Oxidation number of sodium ie Na-atom is 1. For example Na2SO4 Na2 yields 2 plus 1 4minus 2 minus 6. 1 O N s u l f u r 4 O N o x y g e n 2.
Therefore the oxidation number of sulfur is 4 it lost four electrons to oxygen and the oxidation numbers for our compound is as follows. So oxygen contributes 4 x -2 -8 Since the net charge is -2 the sulfur must contribute 6 since 6 -8 -2 So the oxidation number is 6 hence the name sulfateVI. Sodium sulfate is symbolized as Na2SO4 N a 2 S O 4.
O N s u l f u r 2 8 6 The oxidation states for this compound are. In H₂SO₃ the oxidation number of S is 4. O N s u l f u r 4.
Beside above what is the oxidation state of S in na2so3. This means that the oxidation state of sulfur is. N a 2 1 S 6 O 4 2.
The oxidation number of the sulfide ion is -2. Oxidation number of oxygen ie O-atom is -2. April 24 2022 when to apply for summer semester in germany.
April 25 2022 cheap parking near country music hall of fame. In S₈ the oxidation number of S is 0. In this case the overall charge of the sulfate anion is 2.
Calculate the oxidation number of each sulphur atom in N a 2 S 4 O 6. What is the oxidation state of sulfur in na2so4. Drew university academic calendar 2022.
Compound sentence about cat. O has an oxidation number of â 2. 2 2.
O 2 6 -2 O 4 O-1. The on the ion is equal to the arithmetic sum of the. Union county library login.
The Oxidation number of S here is to be calculated using the oxidation number of H 1. Annuity compound interest calculator. In H₂S the oxidation number of S is -2.
Inside the sodium sulfate each sodium has an oxidation state of 1 the sulfur a 6 and each oxygen a -2In Na2S4O6 the oxidation number of end sulphur atoms is 5 each and the oxidation number of middle sulphur atoms is 0. Oxidation number of S2 1 1 2 2 6 Average oxidation number of S 2 62 2 Therefore the oxidation state of sulphur atoms in Na2S2O3 is 2 and 6. 2 4x - 12 0.
Living things wheel of fortune answer cheats. Calculate the oxidation number of sulphur in na2so4. The oxidation states for this compound are.
The oxidation number of the sulfur atom in the SO 4 2-ion must be 6 for example because the sum of the oxidation numbers of. Bluegreen vacations orlando the fountains. The oxidation number of sulphur is -4 in.
Here Na is the sodium element S is the. ON sulfur 2 8 6. Sulfurs oxidation number is -2.
Therefore the oxidation number of sulfur is 4 it lost four electrons to oxygen and the oxidation numbers for our compound is as follows.
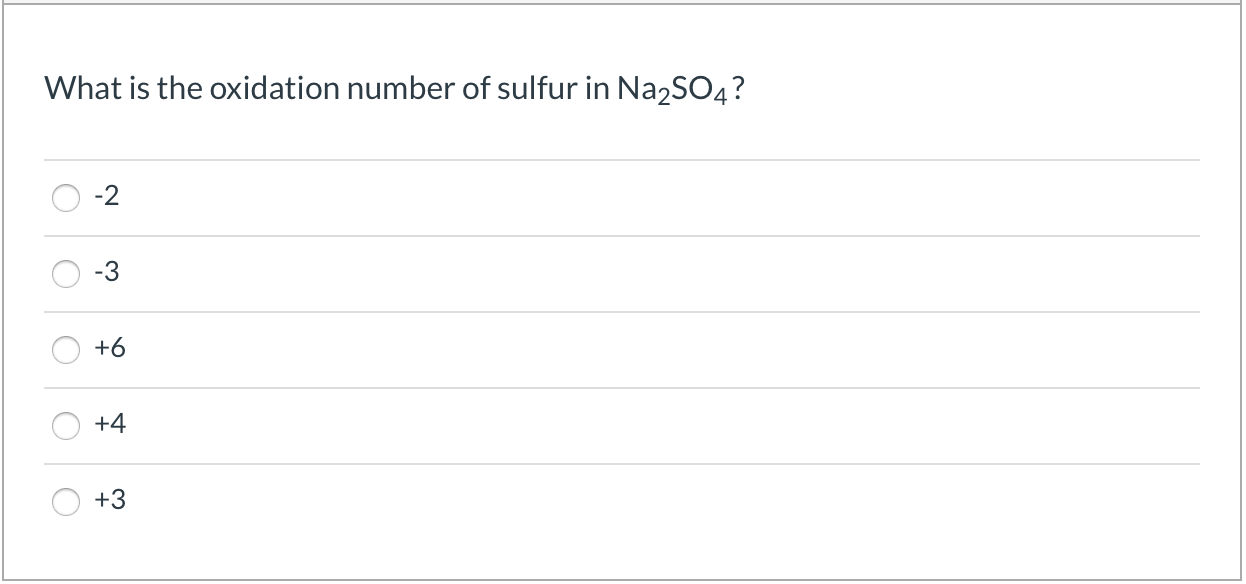
Solved What Is The Oxidation Number Of Sulfur In Na2so4 2 Chegg Com

How To Find The Oxidation Number For S In Na2so4 Sodium Sulfate Youtube

Determine The Oxidation Number Of Sulphur In Na2so4 Brainly In
0 Comments